
Why to use Healaflow?
Thanks to its cross-linked matrix
HealaFlowis made of cross-linkedhyaluronic acidchains associated by covalent bonds through BDDE.
- It offers long lasting presence, up to 6 months, in the surgical plane.
- Its matrix neither splits, nor fragments or dissolves within the aqueous environment
- It shows a cohesive structure, which prevents from migration risks.
What quantity should be injected? Where to place the Healaflow implant?
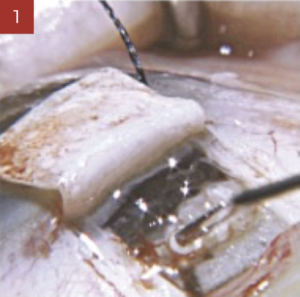
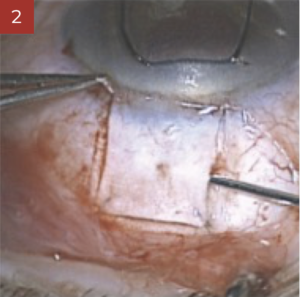
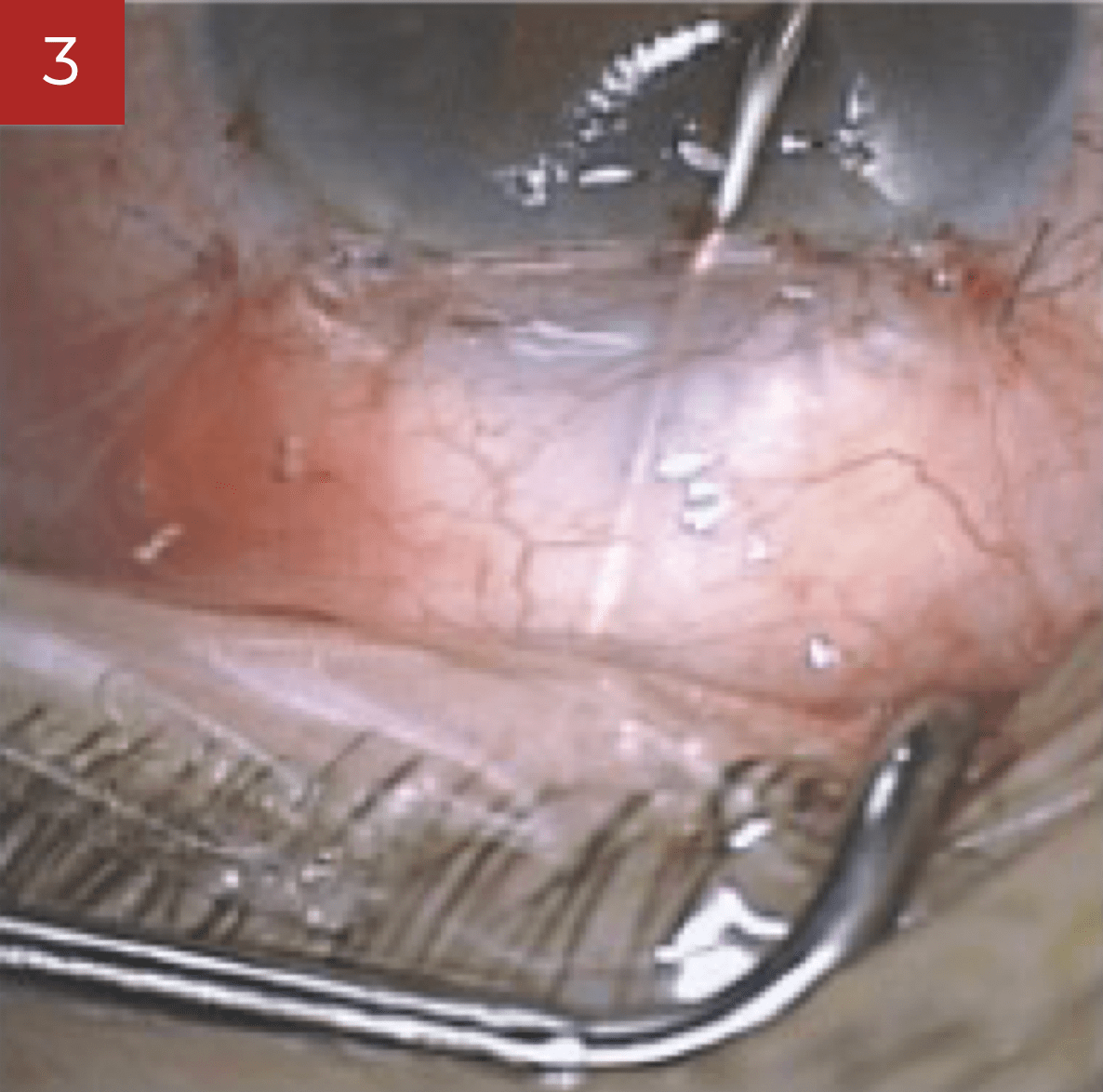
During trabeculectomy, it is convenient to inject the required quantity to lift up the scleral flap sufficiently and to put the sutures under tension. Healaflow shall be placed with care respecting a security distance so as to avoid any risk of involuntary gel injecting into the anterior chamber through the trephination. Any injection under the conjunctiva shall be performed at a safe distance from the limbus, making sure the conjunctival is properly secured (rigorous water tightness). This will help in preventing any risk of postoperative Seidel/bleb leakage.
During deep sclerectomy, the scleral space has to be completely filled with Healaflow, notwithstanding the number of sutures used to tighten the scleral flap. If any injection under the conjunctiva is required, this should follow the same procedure as for trabeculectomy.
In association with the implementation of shunts, stent or tube, Healaflow can be used to fill-up the conjunctiva bleb reasonably.
What to do if Healaflow penetrates the anterior chamber?
Cross-linked sodium hyaluronate is not toxic for ocular tissues, including those of the anterior chambers. In addition, the nature of this implant (a cross-linked gel, thus endowed with a «compact» structure) considerably limits the risk of gel migration into the anterior chamber.
Nevertheless, should the gel be unexpectedly present in the anterior chamber during or after surgery (detected through a deepening of the anterior chamber and slight concavity of the iris), this would require removal of the gel to avoid hypertension risk.
Can Healaflow be simultaneously used with anti fibroblastic (such as MMC, 5FU)? Can we substitute Healaflow for Mitomycin C, and reverse?
The combination of Healaflow and Mitomycin is routinely used to create a synergical action. Indeed, Healaflow is dedicated to long-term preservation of the filtering space. On the other hand it cannot fully control the fibrosis process especially for patients at risk. Thus, Healaflow can be used for keeping the filtering bleb open or acting in the regulation of the aqueous humour outflow. The combination between the structural properties of Healaflow and the pharmacological action of Mitomycin C is beneficial and synergetic.
HA Concentration: 22.5 mg/ml
Volume: 0.6ml
Cross-linking agent: BDDE (1.4 Butanediol Diglycidyl Ether)
Polymer Origin: Non animal- Biofermentation
pH: Physiological pH (7.0)
Osmolarity: isotonic (305 mOsm / kg)
Endotoxin content: < 0.5EU/ml
Protein rate: < 50ppm
Sterilization: Moist heat Sterilization
Contact Us
You can contact us for detailed information about our products

